
Legumain
€990.00
Price excl. shipping costs excl. VAT. For more information, see our shipping policy
SKU: P2020-161 trenzyme
Description
Legumain, also known as asparaginyl endopeptidase (AEP), is a lysosomal cysteine protease that specifically hydrolyzes peptide bonds after asparagine residues, playing a crucial role in various physiological and pathological processes. It contributes to protein turnover and recycling as it is involved in the degradation of proteins within lysosomes. Moreover, it participates in the generation of antigenic peptides for major histocompatibility complex (MHC) class II presentation, thereby contributing to the activation of T lymphocytes. Dysregulation of legumain promotes neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, and is associated with autoimmune diseases, chronic inflammation and various cancer types. Therefore, legumain represents a promising therapeutic target and serves as a potential diagnostic biomarker.
Overview
-
Product Name: Legumain
-
Catalog No.: P2020-158: His-Tag, C-terminal
P2020-159: MBP/His-Tag
P2020-160: Fc/His-Tag
P2020-161: His-Tag, N-terminal
-
RefSeq Links: HGNC:9472; NX_Q99538; NP_001008530.1; NM_005606.6; PDBe 7fqj; UniProt: Q99538
-
Synonyms: Lgmn, LGMN protein, Asparaginyl endopeptidase (AEP), Protease, cysteine 1

Customer Testimonial

“We highly valued the fast and excellent communication and the high flexibility of the team! For any future project, we will preferably entrust in trenzyme’s protein production services.”
Dr. Thore Hettmann
Probiodrug AG, Halle/Saale, Germany
Sequence Information
- Species: Homo sapiens
-
Tags:
P2020-158: His-Tag, C-terminal
P2020-159: MBP/His-Tag
P2020-160: Fc/His-Tag
P2020-161: His-Tag, N-terminal -
Sequence without tags (AA 18-433):
VPIDDPEDGGKHWVVIVAGSNGWYNYRHQADACHAYQIIHRNGIPDEQIVVMMYDDIAYS
EDNPTPGIVINRPNGTDVYQGVPKDYTGEDVTPQNFLAVLRGDAEAVKGIGSGKVLKSGP
QDHVFIYFTDHGSTGILVFPNEDLHVKDLNETIHYMYKHKMYRKMVFYIEACESGSMMNH
LPDNINVYATTAANPRESSYACYYDEKRSTYLGDWYSVNWMEDSDVEDLTKETLHKQYHL
VKSHTNTSHVMQYGNKTISTMKVMQFQGMKRKASSPVPLPPVTHLDLTPSPDVPLTIMKR
KLMNTNDLEESRQLTEEIQRHLDARHLIEKSVRKIVSLLAASEAEVEQLLSERAPLTGHS
CYPEALLHFRTHCFNWHSPTYEYALRHLYVLVNLCEKPYPLHRIKLSMDHVCLGHY
Product Information
-
Expression Host: HEK293
-
Formulation: 20 mM Tris, 20 mM NaCl, 5 mM DTT; pH 7.5
- Format: Liquid, stored and shipped at -80° C
-
Purity: > 85 % as determined by SDS-PAGE
- Application: Digestion proteomics
Background Information
Legumain functions as lysosomal cysteine protease, specifically hydrolyzing peptide bonds after asparagine residues. Thereby, legumain plays a crucial role in the degradation of intracellular proteins.
Structurally, it contains a highly conserved His148-Gly-spacer-Ala-Cys189 motif, which is characteristic for members of the CD clan of cysteine proteases, such as caspase-1, clostripain and gingipain R. Legumain belongs to the C13 family of peptidases and is synthesized as inactive zymogen consisting of a N-terminal pro-peptide (Val18-Asp25), the cysteine protease domain (Gly26-Asn323) and a C-terminal pro-domain (Asp324-Tyr433). To become active, legumain requires autoproteolytic cleavage resulting in the release of both pro-peptides. Particularly, a pH shift to below 5.5 leads to autocatalytic removal of the C-terminal pro-domain and a further decrease in pH to approximately 4.0 removes the N-terminal pro-peptide, thus releasing the active protease.
Legumain is responsible for the proteolysis of endocytosed proteins, generating antigenic peptides that bind to class II major histocompatibility complex (MHC) molecules with high affinity. In addition, the proteolytic activity of legumain promotes the dissociation of the invariant chain (Ii), which occupies the peptide-binding cleft of class II MHC molecules to prevent peptide binding within the endoplasmic reticulum (ER). Processing and dissociation of the Ii in lysosomes enables association of processed, high affinity peptides that are displayed on the surface of antigen presenting cells (APCs). This results in antigen recognition by CD4+ T lymphocytes and their activation. Hence, legumain plays an essential role in the regulation of adaptive immune responses.
In cancer, increased legumain expression has been associated with tumor progression, invasion, and metastasis, due to increased degradation of the extracellular matrix. Additionally, overexpression of legumain has been implicated in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, where its dysregulation contributes to the accumulation of misfolded proteins.
Additional information Legumain His-Tag, C-terminal
|
SDS-PAGE/Coll. Coomassie |
Histogram of marked lane in gel picture |
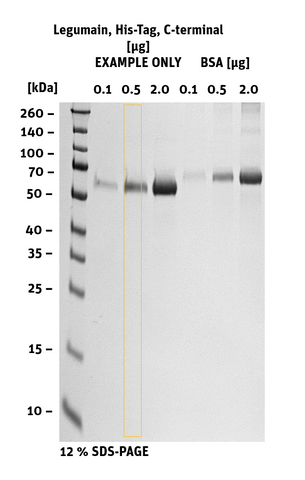 |
 |
Additional information Legumain MBP/His-Tag
|
SDS-PAGE/Coll. Coomassie |
Histogram of marked lane in gel picture |
 |
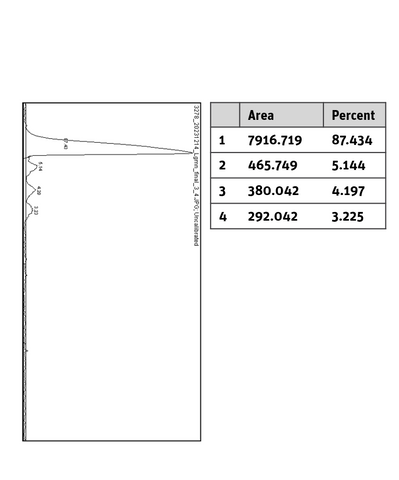 |
Additional information Legumain Fc/His-Tag
|
SDS-PAGE/Coll. Coomassie |
Histogram of marked lane in gel picture |
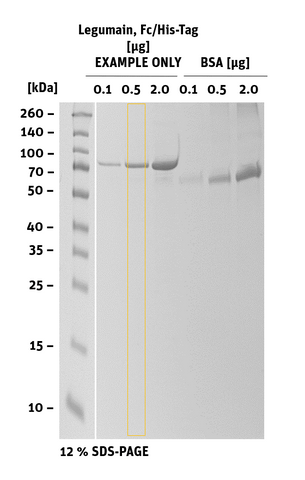 |
 |
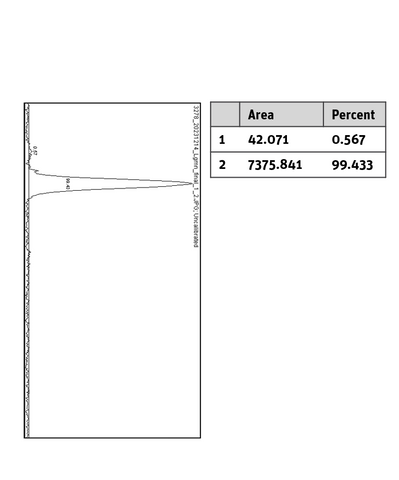
Additional information Legumain His-Tag, N-terminal
|
SDS-PAGE/Coll. Coomassie |
Histogram of marked lane in gel picture |
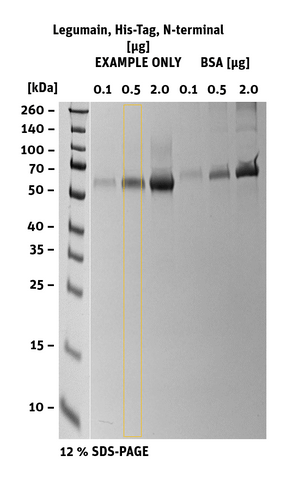 |
 |



